What are the components of HDPE that make it possible to convert into oil?
High-density polyethylene (HDPE) is a type of polymer that is not easily converted back into its monomeric form through a simple chemical process. However, there are certain methods available to convert HDPE into oil-like substances. The process typically involves thermal decomposition or pyrolysis, which breaks down the polymer chains into smaller hydrocarbon molecules.
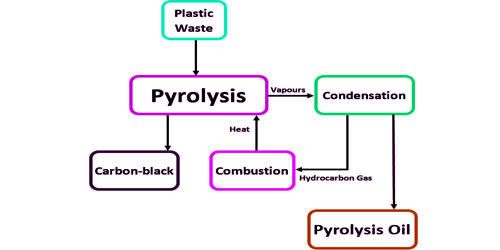
During the pyrolysis of HDPE, the following components may be obtained:
-
Hydrocarbons: The pyrolysis process breaks down the long hydrocarbon chains of HDPE into shorter hydrocarbon molecules. These hydrocarbons can include various forms of alkanes, alkenes, and aromatic compounds.
-
Gases: The thermal decomposition of HDPE can produce gases such as methane, ethylene, propylene, and other lighter hydrocarbons. These gases can be collected and used as fuel or further processed.
-
Liquid Fraction: The pyrolysis process can yield a liquid fraction, often referred to as pyrolysis oil or plastic oil. This liquid can contain a mixture of hydrocarbon compounds with a wide range of boiling points. It can be further refined or processed to obtain usable fuels or chemical feedstocks.
-
Solid Residue: After the pyrolysis process, there is usually a solid residue left behind, often referred to as char or carbon black. This solid residue can have applications in various industries such as energy production or as a filler in the production of other materials.
It's important to note that the conversion of HDPE into oil or oil-like substances through pyrolysis is typically carried out on a large scale in specialized facilities. The resulting products can vary depending on the specific process parameters, temperature, and duration of the pyrolysis process.
What is pyrolysis?
Pyrolysis is a chemical process that involves the thermal decomposition of organic materials in the absence of oxygen or with limited oxygen supply. It is a form of thermolysis, where the material is subjected to high temperatures in the range of 400 to 800 degrees Celsius (752 to 1472 degrees Fahrenheit) in a controlled environment.
During pyrolysis, the organic material, such as biomass, plastic, or rubber, undergoes a series of complex reactions due to the high heat. These reactions break down the large complex molecules into smaller molecules, gases, and char or solid residues. The absence of oxygen prevents combustion and allows for the production of valuable byproducts.
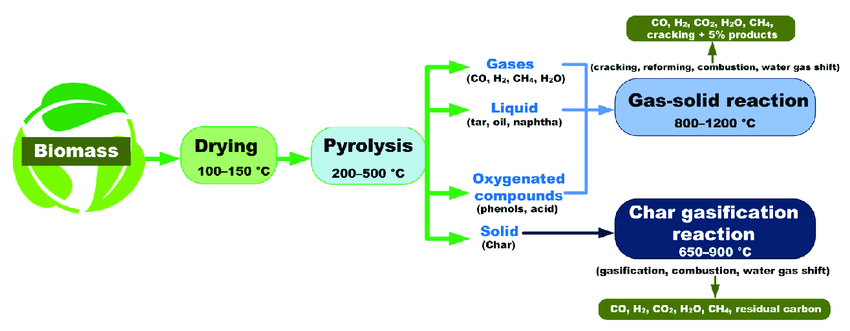
Pyrolysis typically occurs in three stages:
-
Drying and Devolatilization: Initially, the material is heated, and any moisture or volatile components present in the material are driven off as vapor. This stage involves the release of water, light gases, and volatile organic compounds.
-
Gasification: As the temperature increases, the remaining organic components undergo further decomposition, leading to the production of gases. These gases often include carbon monoxide, hydrogen, methane, and other hydrocarbon compounds. The gas composition depends on the nature of the feedstock used in the pyrolysis process.
-
Char Formation: The solid residue or char left behind after the volatile components have been driven off is a carbon-rich material. It consists of carbonaceous compounds, ash, and other inorganic materials present in the original feedstock. The properties of the char can vary depending on the type of material being pyrolyzed.
The byproducts of pyrolysis, such as gases, bio-oils, and char, can have various applications. The gases can be used as fuel or further processed for energy generation. Bio-oils, also known as pyrolysis oils, can be refined and used as a renewable source of liquid fuels or as feedstock for the production of chemicals. Char can be utilized as a solid fuel, as a soil amendment, or in the production of activated carbon.
Pyrolysis offers a means to convert organic materials into valuable products, reduce waste, and promote resource efficiency. It has applications in waste
management, biomass conversion, and the recycling of certain types of plastics.
:read more